(Inactivated virus)
A. Product appearance and performance parameters
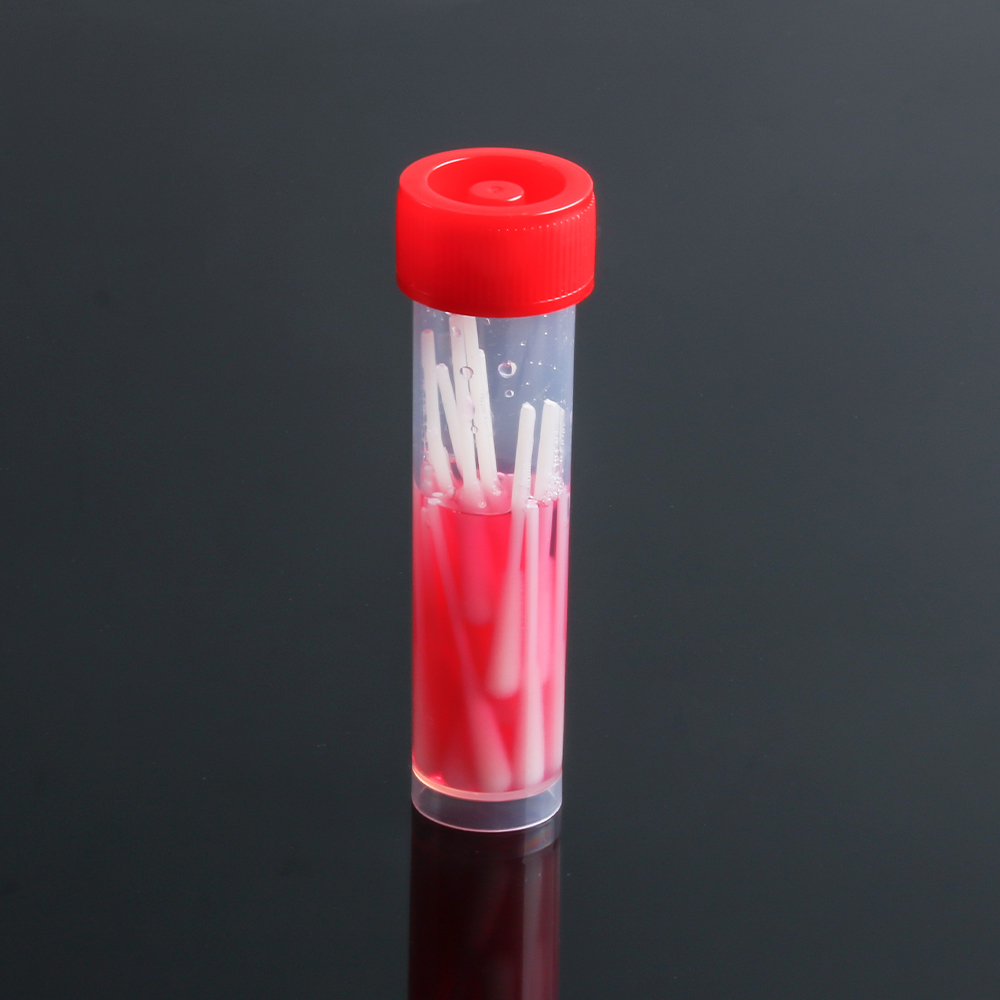
①, virus sampling tube.
Height is 100mm, diameter 16mm
Tube body material PP (polypropylene), can withstand -197 ℃, low temperature does not break, 125 ℃ high temperature and high pressure does not deform
Tube cover material PE (high-density polyethylene), to ensure a close combination with the body of the tube
The inner bottom of the tube is tapered to allow for centrifugation and shaking. Conical bottom with skirt, all tubes can stand on the surface of the biological safety cabinet without the use of test tube racks and so on
②. Sampling swabs.
Length 150mm
Swab rod for PP material (polypropylene), inert material, submerged in the sampling fluid will not have foreign matter precipitation.
Swab head for fiber material, does not contain cotton material will have non-saturated fatty acids, will not interfere with the accuracy of PCR nucleic acid detection.
Second, the product packaging
Packaging specifications: 20 people / box
Each person includes 1 virus sampling tube (containing virus preservation solution), 1 individually wrapped sterile sampling swab, and 1 biosafety bag.
Third, the use of products
①, the collection of influenza virus, hand, foot and mouth virus, measles rubella virus samples
②, the collected virus samples can be stored for a short time at 2-8 ℃ temperature conditions (within 72 hours.)
③, the collected virus samples are used for nucleic acid detection and virus isolation
IV. Biosafety protection aspects
①、The double anti-leakage design of own knowledge product and patented tube cover to avoid leakage of infectious virus preservation solution after sampling.
The O-ring seal on the inner wall of the cap ensures the sealing of the sampling tube without external extrusion.
The combination of cap and tube body has higher pressure strength. Ensure the seal when the sampling tube is squeezed by external force.
②. Each package comes with a self-sealing biosafety bag. The sampling tube can be transported in it after sampling. More secure.
V. Product performance
①、High positive rate of nucleic acid detection
24 hours after sample collection, the minimum detection line for positive nucleic acid test can be as low as 1*10²cell/mL (the virus titer refers to the virus low at the time of sample collection, the real time virus low after sample collection in the conventional onset population is 1*10^6cell/mL)
②. Product can be stored at room temperature
Optimized product formulation, selected room temperature stable components. The product can be stored at room temperature
The liquid chromatogram of the product stored for 7d and 375d shows that there is no significant decrease in each component of the product.
③. Good inhibition effect on mold
The selection of room temperature stable antibiotics to inhibit mold, to avoid the selection of similar amphotericin B, mycotoxin and other room temperature unstable antibiotics occurred after sampling the virus preservation solution moldy, hairy and other phenomena.
Sixth, the product qualification
All through the EU CE, the United States FDA, the United States EUA, China NMPA, SGS, TUV and other field certification, can export trade
Domestic also has the registration certificate
Translated with www.DeepL.com/Translator (free version)